Image of the Month - February 2020
(Photo credit Esther Colonnese)
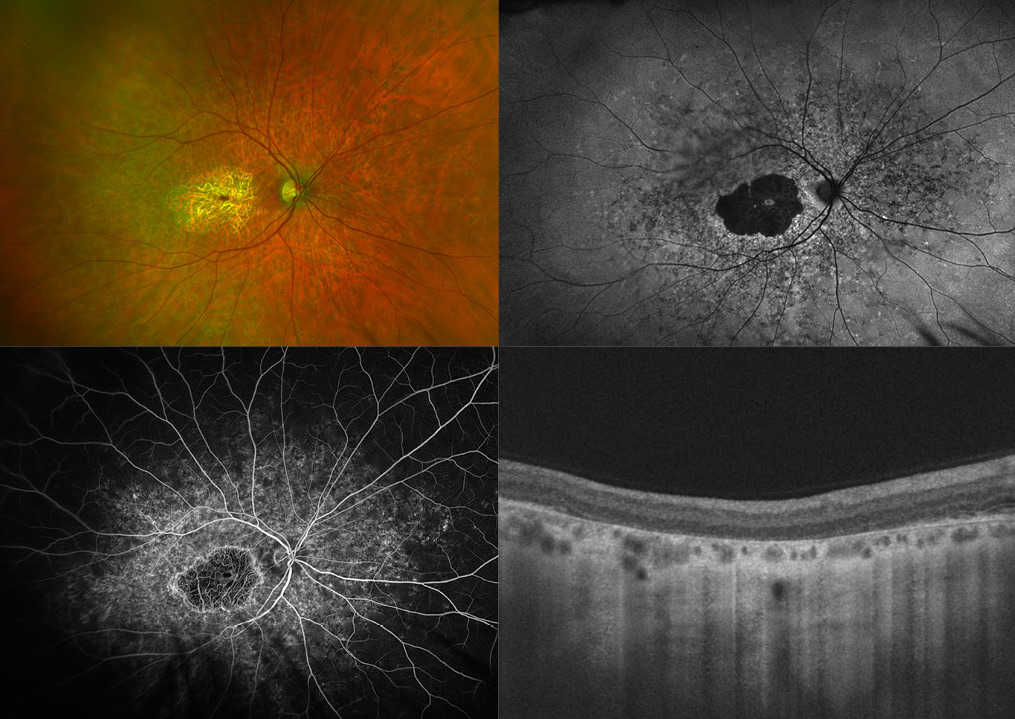
This multi-modal image illustrates a patient with a retinal dystrophy. The widefield fundus photo (top left) demonstrates macular atrophy with underlying larger choroidal vessels visible. Autofluorescence (top right) shows central hypo-autofluorescence in the macular area corresponding to a loss of the retinal pigment epithelium (RPE), with hyper-autofluorescent edges indicating increased metabolic demand of peripheral RPE cells. There are radiating lines of hypo-autofluorescence towards the periphery correlating with centripetal disease extension. There are hyper-autofluorescent spots in the periphery as well, corresponding to accumulations on the RPE. Note the remaining normal autofluorescence within the fovea, which correlates well with a small island of preserved functional central vision. The Fluorescein angiogram (bottom left) shows a central window defect as well as peripheral staining. The choroid is notably dark. Optical coherence tomography (bottom right) demonstrates loss of the RPE and photoreceptors. The fellow eye is not shown here but had a similar appearance. The patient noted poor vision his entire life and denied a family history of retinal disease. This series of images depicts the overlapping characteristic features of several diseases. The differential diagnosis includes Stargardt's disease, Central areolar choroidal dystrophy, North Carolina macular dystrophy as well as Toxoplasmosis infection. Genetic testing was obtained and revealed two disease causing mutations in the ABCA4 gene. Taken into clinical context, the most likely diagnosis is advanced Stargardt's disease. The macular atrophy is a sign of advanced disease, yet, the dark choroid on Fluorescein angiogram and Pisciform flecks best depicted on Autofluorescence, are helpful signs to clinch the diagnosis. This case illustrates multiple paradigm shifts in our medical landscape. First, multi-modal retinal imaging has become an indispensable tool in our daily retina clinics. Next, genetic testing is at the intersection of mainstream clinical practice and is in many ways changing not only how we diagnose, but also how we define diseases. In a few years, we may rename Stargardt's disease ABCA4-associated maculopathy as more accurate. Lastly, gene modifying therapy has become a reality. Ophthalmology recently became the first field in all of medicine with an FDA approved gene-therapy, Voretigene neparvovec (Luxturna®) for RPE-65 associated Leber's congenital amaurosis. While ABCA4 is a more difficult gene-target to treat, multiple prospective clinical trials for Stargardt's are underway.

